Current Research |
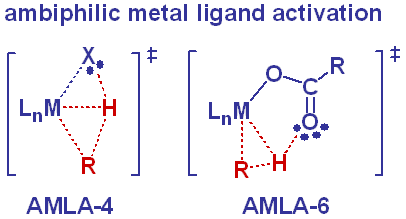 | C–H Activation and Functionalisation Hydrocarbons are very abundant and extremely cheap and so would make ideal chemical feedstocks – were it not for their notoriously low chemical reactivity. However, facile C–H activation can occur at electron deficient metal centres in the presence of a chelating base, such as acetate. In collaboration with Prof. Dai Davies at the University of Leicester We have developed the concept of the ambiphilic metal-ligand activation (AMLA) mechanism, whereby an agostic interaction renders the C–H bond susceptible to an intramolecular deprotonation. Current efforts in this area focus on the catalytic functionalisation of C–H bonds in collaboration with Prof. Igor Larrosa of the University of Manchester. |
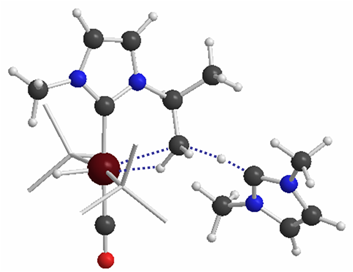 | Transition-Metal-Main Group Heterobimetallic Chemistry N-heterocyclic carbenes (NHCs) are popular ligands in organometallic chemistry and homogeneous catalysis, largely due to their strong donor capacity and the idea that NHCs are less prone to ligand degradation processes compared to related phosphine complexes. However, experimental studies by have shown that NHC ligands are often non-innocent and can undergo a variety of intramolecular bond activaton reactions. We are interested in understanding the factors that control these processes, so that improved catalysts can be designed. However, the cleavage of normally inert bond has its own intrinsic interest and the study of NHC ligands has provided insight into fundamental bond activation processes, including C–H, C–N and even C–C activation. |
Ruthenium N-Heterocyclic Carbene (NHC) Complexes as Hydrodefluorination Catalysts in collaboration with Prof. Mike Whittlesey at the University of Bath NHC ligands often confer enhanced reactivity on metal complexes. An example is the hydrodefluorination of C6F5H to give 1,2-C6F4H2 catalysed by [Ru(H)2(CO)(NHC)(PR3)2] species. Calculations show this unusual ortho-selectivity arises from a nucleophilic attack mechanism where the hydride ligand (and not the metal) acts as the reacting species. The use of N-aryl substituted NHCs is the key to this unusual selectivity as they provide a 'pocket' that can stabilise a highly negatively charged fluoride centre that is formed upon C–F bond cleavage. Current research aims to extend this reactivity to lower fluorinated species and to different nucleophiles other than hydride. | 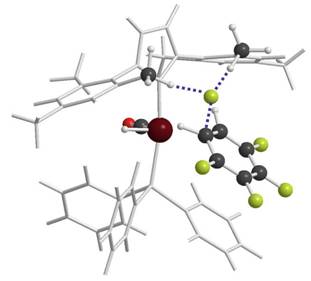 |
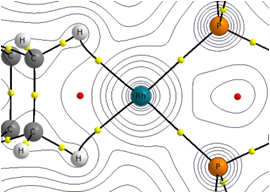 | Transition Metal Alkane sigma Complexes and Amineborane Chemistry in collaboration with Prof. Andy Weller at the University of York Calculations have provided complementary structural and spectroscopic data (13C, IR) to support of the first example of a crystallographically characterized σ-alkane complex. This species, [Rh(nBu2PCH2CH2PnBu)(norbornane)]+ features norbornane ligand bound in a bidentate fashion to a Rh+ centre and is formed via the crystal-to-crystal reaction of the diene precursor with hydrogen gas. Further computational work will aim to highlight the factors that stabilize the Rh-alkane interaction in order to design more stable examples that may persist even in solution. Electronic structure analysis tool such as atoms-in-molecules and natural bond order methods will be employed. We are also interested in the mechanism of amineborane dehydrocoupling at Rh and Ir {ML2}+ fragments and controlling the subsequent formation of {H2BNR2)n polymers, the inorgaic equivalent to polyalkanes. |
Heriot-Watt University, Institute of Chemical Sciences, Edinburgh, Scotland, UK EH14 4AS
